Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
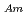 ) and Cm (
) and Cm (
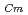 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:15 Percentile:52.81(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be  99% by optimizing separation conditions.
99% by optimizing separation conditions.
 -tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cell
-tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya; Hotoku, Shinobu; Kawasaki, Tomohiro*; Sagawa, Hiroshi*; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(1), p.27 - 37, 2019/00
Times Cited Count:21 Percentile:65.06(Chemistry, Multidisciplinary)A continuous counter-current experiment using TDdDGA was performed using mixer-settler extractors installed in a hot cell. Nitric acid containing minor actinides (MAs: Am and Cm), rare earths (REs: Y, La, Nd, and Eu), and other fission products (Sr, Cs, Zr, Mo, Ru, Rh, and Pd) was fed to the extractor. TDdDGA effectively extracted MAs and REs from the feed, while other fission products were barely extracted. The extracted MAs and REs were back-extracted by bringing them in contact with 0.02 mol/dm nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were
nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were  98% and
98% and  86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
Arai, Yasuo; Pillon, S.*
Proceedings of International Conference ATALANTE 2004 Advances for Future Nuclear Fuel Cycles (CD-ROM), 9 Pages, 2004/06
no abstracts in English
Ozaki, Takuo; Kimura, Takaumi; Onuki, Toshihiko; Yoshida, Zenko; Francis, A. J.
Environmental Toxicology and Chemistry, 22(11), p.2800 - 2805, 2003/11
Times Cited Count:21 Percentile:45.2(Environmental Sciences)no abstracts in English
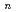 (
( =1-4) by all-electron Dirac-Hartree-Fock calculations
=1-4) by all-electron Dirac-Hartree-Fock calculationsMochizuki, Yuji*; Tatewaki, Hiroshi*
Journal of Chemical Physics, 118(20), p.9201 - 9207, 2003/05
Times Cited Count:6 Percentile:18.14(Chemistry, Physical)no abstracts in English
Tatewaki, Hiroshi*; Mochizuki, Yuji*
Theoretical Chemistry Accounts, 109(1), p.40 - 42, 2003/01
Times Cited Count:23 Percentile:52.43(Chemistry, Physical)no abstracts in English
 Cm-
Cm- Pu oxide and mononitride
Pu oxide and mononitrideTakano, Masahide; Ito, Akinori; Akabori, Mitsuo; Ogawa, Toru; Numata, Masami; Kizaki, Minoru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.842 - 845, 2002/11
no abstracts in English
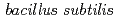 and halobacterium salinarum
and halobacterium salinarumOzaki, Takuo; Gillow, J. B.*; Francis, J. A.*; Kimura, Takaumi; Onuki, Toshihiko; Yoshida, Zenko
Journal of Nuclear Science and Technology, 39(Suppl.3), p.950 - 953, 2002/11
no abstracts in English
 O)
O)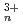 (n=1, 2, 4, 6) by all-electron Dirak-Hartree-Fock calculations
(n=1, 2, 4, 6) by all-electron Dirak-Hartree-Fock calculationsMochizuki, Yuji*; Tatewaki, Hiroshi*
Journal of Chemical Physics, 116(20), p.8838 - 8842, 2002/05
Times Cited Count:16 Percentile:45.25(Chemistry, Physical)no abstracts in English
Kato, Hiroshige*; Mine, Tatsuya*; Mihara, Morihiro; Oi, Takao; Honda, Akira
JNC TN8400 2001-029, 63 Pages, 2002/01
Cementitious materials will be used for the TRU waste repository as a component of engineered barrier system. The distribution coefficients which represent the retardation of radionuclides migration for the cementitious materials would be one of the important parameter for the safety assessment. The much information of radionuclide sorption onto the cementitious materials has been accumulated through the study in the world. Therefore it is necessary to compile the information and Kd of the radionuclides reported in previous studies. In this report, the Kd of the important radionuclides, such as C, Ni, Se, Sr, Zr, Nb, Mo, Tc, Sn, I, Cs, Sm, Pb, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, for the cementitious materials were compiled as the Sorption Database (SDB). For radionuclides to be sensitive to the redox potential, e.g. Se, Tc, Pa, U, Pu and Np, some Kds measured under the controlled atmosphere had been reported, and few Kds measured under the controlled redox potential had been reported. For Se, Mo, Sm, Cm and Ac, the distribution coefficients had not been reported, therefore distribution coefficients of Se and Mo for OPC (Ordinary Portland Cement) pastes were measured by batch sorption experiments and these data were added into the SDB.
Kimura, Takaumi; Nagaishi, Ryuji; Kato, Yoshiharu; Yoshida, Zenko
Radiochimica Acta, 89(3), p.125 - 130, 2001/05
Times Cited Count:66 Percentile:96.81(Chemistry, Inorganic & Nuclear)no abstracts in English
Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Ogawa, Toru; Numata, Masami; Okamoto, Hisato
Journal of Nuclear Materials, 294(1-2), p.24 - 27, 2001/04
Times Cited Count:13 Percentile:66.91(Materials Science, Multidisciplinary)no abstracts in English
Osaka, Masahiko; Koyama, Shinichi; Mitsugashira, Toshiaki; Morozumi, Katsufumi; Namekawa, Takashi
JNC TN9400 2000-058, 49 Pages, 2000/04
The analytical technique for Cm contained in a MOX FUEL was developed and analysis of Cm contained in irradiated fuel of experimental fast reactor "JOYO" was carried out, to contribute to evaluation of transmutation characteristics of MA nuclide in the fast reactor. The procedure of ion-exchange separation of Cm with nitric acid-methanol mixed media essential for the isotopic analysis in irradiated MOX fuel was adopted considering for being rapid and easy. The fundamental test to grasp separation characteristics of this procedure, such as Cm elution position and separation capacity between Cm and Am or Eu, was carried out. ln applying this procedure to the analysis of Cm contained in actual specimen, separation condition was evaluated and optimized, and the procedure consist of impurity removal and Am removal process was devised. This procedure resulted in high recovery rate of Cm and high removal rate of Am and impurity which becomes a problem in sample handling and mass-spectrometry such as Eu and Cs. The Cm separation test from irradiated MOX fuel was carried out using this technique, and Cm isotopic ratio analysis was enabled. The analytical technique for Cm contained in irradiated MOX fuel was established using the procedure of ion-exchange separation with nitric acid-methanol mixed media. The analysis of Cm contained in irradiated MOX fuel of experimental fast reactor "Joyo" was carried out. As a result, it was revealed from measured data that Cm content rate was 1.4 4.0
4.0 lO
lO atom%, small amount of
atom%, small amount of 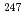 Cm was generated and Cm isotopic ratio was constant above burn-up 60GWd/t.
Cm was generated and Cm isotopic ratio was constant above burn-up 60GWd/t.
Yaita, Tsuyoshi
Proceedings of International Youth Nuclear Congress 2000 (CD-ROM), 4 Pages, 2000/00
no abstracts in English
; ; Ishiguro, Katsuhiko; Nakajima, Kunihiko*;
JNC TN8400 99-087, 41 Pages, 1999/11
Corrosion of the carbon steel overpack leads to a volume expansion since the specific gravity of corrosion products is smaller than carbon steel. The buffer material is compressed due to the corrosive swelling, reducing its thickness and porosity. On the other hand, Buffer material may be extruded into fractures of the surrounding rock and this may lead to a deterioration of the planned functions of the buffer, including retardation of nuclides migration and colloid filtration. In this study, the sensitivity analyses for the effect of volume expansion and intrusion of the buffer material on nuclide migration in the engineering barrier system are carried out. The sensitivity analyses were performed on the decrease in the thickness of the buffer material in the radial direction caused by the corrosive swelling, and the change in the porosity and dry density of the buffer caused by both compaction due to corrosive swelling and intrusion of buffer material. As results, it was found the maximum release rates of relatively shorter half-life nuclides from the outside of the buffer material decreased for taking into account of a volume expansion due to overpack corrosion. On the other hand, the maximum release rates increased when the intrusion of buffer material was also taking into account. It was, however, the maximum release rates of longer half-life nuclides, such as Cs-137 and Np-237, were insensitive to the change of buffer material thickness, and porosity and dry density of buffer.
Ashida, Takashi; ; Sato, Haruo; ; Kitamura, Akira; Kawamura, Kazuhiro
JNC TN8400 99-083, 63 Pages, 1999/11
Studies on the chemical and migration behaviour of radionuclides were carried out in the Quantitative Assessment Radionuclide Migration Experimental Facility (QUALITY)for assuring the relaiability and for improving the propriety of data concerning nuclide migration used in the Second Progress Report for the geoloical disposal of high-level radioactive waste. Five studies for solubility, sorption and diffusion concerning nuclide migration were carried out. The overview of each study and the result is as follows: (1)Study on Effect of Carbonate on Np Solubility. Solubilities of Np(IV) were measured as functions of pH and carbonate concentration under reducing conditions. The obtained data could be well described by considering two hydroxo-carbonate complexes, and those stability constants were estimated and compared with the literature data. Consequently, the data obtained in this study were similar to the literature data. (2)Study on Effect of Carbonate on Np Sorption on Bentonite. Distribution coefficients (Kd) of Np(IV) on smectite were measured as a function of carbonate concentration. The obtained Kd values were approximately constant over the carbonate concentration (total carbon concentration 0.04-0.15M). The results of desorption tests by 1M KCl and HCl at the end of sorption experiments showed two different desorption behaviour; Np(IV) was well removed by HCl for the experiments in low carbonate concentration and by KCl for those in high carbonate concentration. (3)Distribution Coefficient Measurements for Cs, Pb and Cm on Rocks. Distribution Coefficients for Cs, Pb and Cm on Japanese major rocks (basalt, mudstone, sandstone, granodiorite and tuff) were measured as a function of ionic strength. The obtained Kd values were either the same orders or higher compared with data used to both fresh and saline groundwater systems in the Second Progress Report. This indicates that the Kd data used in the Second Progress Report are either proper or conservative. ...
Rai, D.*; Rao, L.*; Weger, H. T.*; GREGORY R.CHOPPI*; Yui, Mikazu
JNC TN8400 99-010, 95 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Pu(III), Am(III), and Cm(III) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(III) species are lacking, the data were selected based on chemical analogy to other trivalent actinides. In this study, the Pitzer ion-interaction model is mainly used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Nakagawa, Tsuneo; Takano, Hideki; Hasegawa, Akira
NEA/WPEC-8, p.1 - 116, 1999/00
no abstracts in English